DIFFUSION WEIGHTED IMAGING – BACKGROUNDS AND TECHNICALS
On this page we would like to introduce you to some background scares about the technique used to create the BrainPics fiber images of the human brain.
All fiber images you see on our BrainPics website have been calculated based on diffusion weighted magnetic resonance images. Diffusion weighted magnetic resonance imaging is a special magnetic resonance imaging (MRI) technique. MRI measurements are not harmful to the human body, as MRI measurements do not work with high-energy radiation, as X-ray methods / CT imaging, but with strong magnetic fields and electromagnetic radiation in the harmless area for the human organism. This is the main reason why we can also make you the special offer to create fiber pictures of your own brain without having to expose yourself to any health risks or harmful X-rays.
SHORT INTRODUCTION TO THE DIFFUSION WEIGHTED IMAGING
 The human brain consists mainly of grey and white brain matter. The cortex accounts for the largest proportion of the grey matter and consists mainly of nerve cell bodies, which form various functional centers. The most important functional centers are, for example, the primary motor and the primary sensory areas. The different cortex areas are responsible for different tasks and are functionally strongly connected with each other.
The human brain consists mainly of grey and white brain matter. The cortex accounts for the largest proportion of the grey matter and consists mainly of nerve cell bodies, which form various functional centers. The most important functional centers are, for example, the primary motor and the primary sensory areas. The different cortex areas are responsible for different tasks and are functionally strongly connected with each other.
This crosslinking of the functional areas takes place via nerve fibers. The white brain substance consists mainly of such connections or nerve fibers and thus forms the structural network of the human brain.
Diffusion weighted imaging allows conclusions to be drawn as to how this white matter is structured and organised. Diffusion-weighted imaging is the only technique that allows the anatomical structure and architecture of the entire human neural network to be explored in-vivo, non-invasively and quantitatively!
BROWNIAN MOTION (DIFFUSION) OF WATER MOLECULES
In liquids, molecules, such as water molecules in a glass of water, are constantly in motion due to their thermal energy. Einstein and Smoluchowski have found that the path a molecule travels in a liquid can be calculated as follows:
Einstein-Smoluchowski formula: R=√6*D*Δ
 Here, Δ is the time that the molecule can diffuse and D is the so-called diffusion coefficient. The diffusion coefficient is a measure of particle mobility and characteristic for any liquid (for a certain temperature).
Here, Δ is the time that the molecule can diffuse and D is the so-called diffusion coefficient. The diffusion coefficient is a measure of particle mobility and characteristic for any liquid (for a certain temperature).
Diffusion weighted imaging is now able to measure, quantify and calculate the diffusion coefficient of this so-called brownian movement of water molecules in any spatial direction.
If molecules, as shown in the figure on the left, can diffuse freely in the ocean, for example, we are talking about so-called free, isotropic diffusion. According to the above formula, the path in the square that a molecule travels increases linearly with the diffusion time. The molecules can spread equally strongly in each spatial direction, resulting in a spherical diffusion profile.
 But if we look at a nerve fiber, we can think of it like a water hose. In a hose, the water flows along the hose much stronger than vertically to the hose walls. Likewise, diffusion in a nerve fiber is restricted perpendicular to the fiber, while along the nerve fiber the diffusion is more or less free. In this case, we are talking about so-called hindered or anisotropic diffusion. This restriction of diffusion in one or more spatial directions leads to an elongated, cigar-shaped diffusion profile.
But if we look at a nerve fiber, we can think of it like a water hose. In a hose, the water flows along the hose much stronger than vertically to the hose walls. Likewise, diffusion in a nerve fiber is restricted perpendicular to the fiber, while along the nerve fiber the diffusion is more or less free. In this case, we are talking about so-called hindered or anisotropic diffusion. This restriction of diffusion in one or more spatial directions leads to an elongated, cigar-shaped diffusion profile.
If the diffusion is now limited in one or more spatial directions, this direction-dependent diffusion strength can be described with a so-called tensor, a symmetric 3×3 matrix. This tensor information finally allows conclusions to be drawn on the underlying tissue.
CALCULATION OF THE DIFFUSION TENSOR
 To calculate the diffusion tensor, the strength of the diffusion must be measured and quantified in at least 6 different spatial directions. From this data, a symmetric diffusion tensor can then be calculated using a linear equation system for each point in the image. The tensors can now be represented as spheroid. The main axis of the tensors indicates the direction in which the diffusion is most pronounced at the underlying location. Analogous to the water hose, this direction is parallel to the underlying nerve fiber.
To calculate the diffusion tensor, the strength of the diffusion must be measured and quantified in at least 6 different spatial directions. From this data, a symmetric diffusion tensor can then be calculated using a linear equation system for each point in the image. The tensors can now be represented as spheroid. The main axis of the tensors indicates the direction in which the diffusion is most pronounced at the underlying location. Analogous to the water hose, this direction is parallel to the underlying nerve fiber.
If, for example, one now presents the tensors in each pixel in a coronary section, you can already recognize certain structures and patterns, as shown in the graphic on the right. In the enlarged area you can see in blue running from bottom to top, for example, the corticospinal tract and in red from left to right the region of the Corpus Callosum. From this representation, it is no longer so difficult to imagine how a fiber tracking algorithm can reconstruct nerve fibers.
1) Basser et al., Biophysical Journal 1994
2) Pierpaoli et al., Radiology 1996
RECONSTRUCTION OF FIBERS
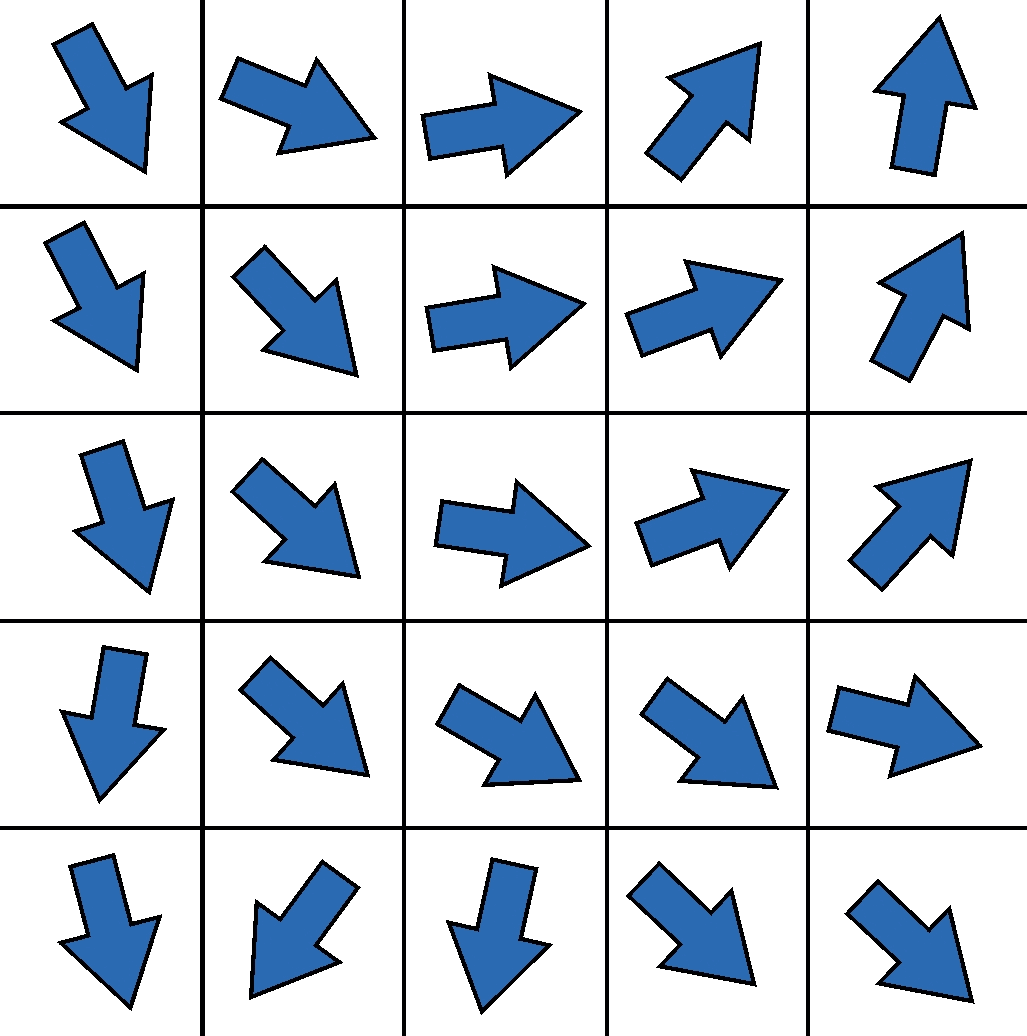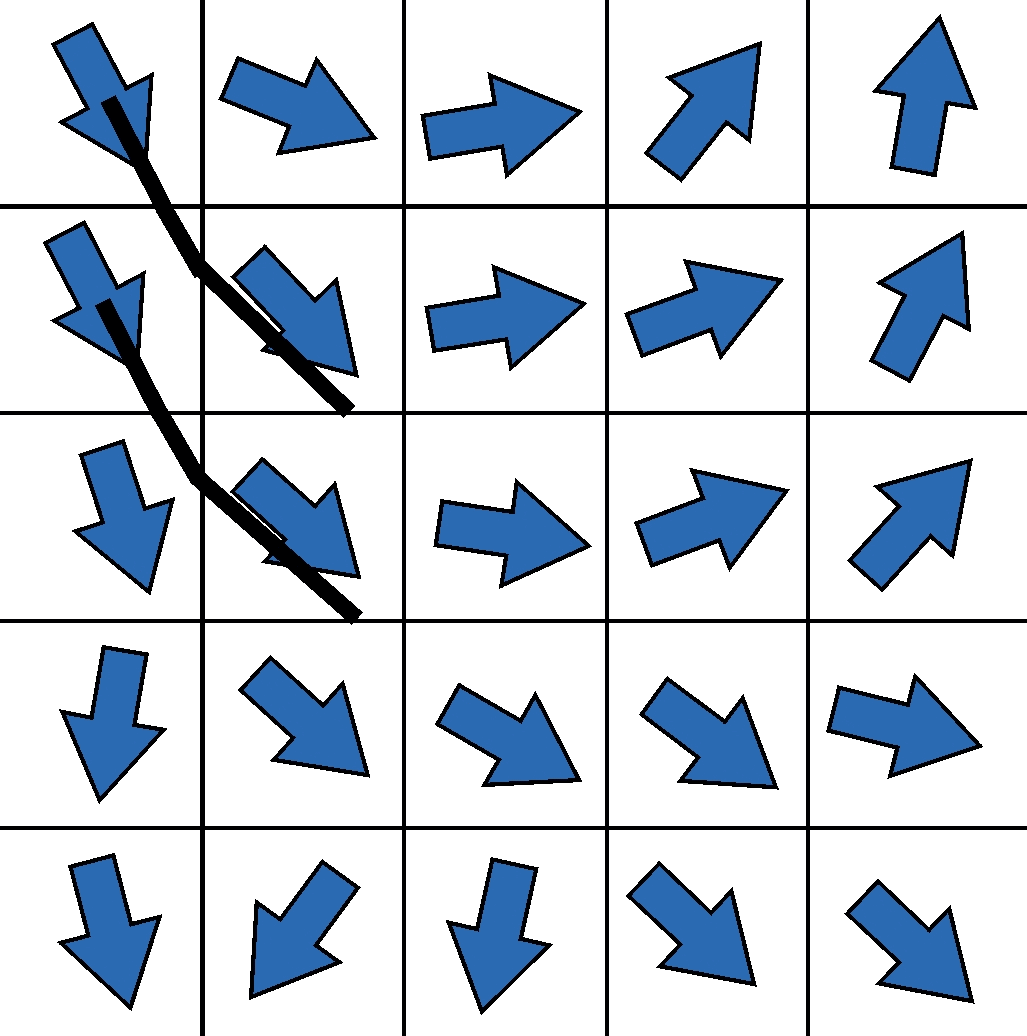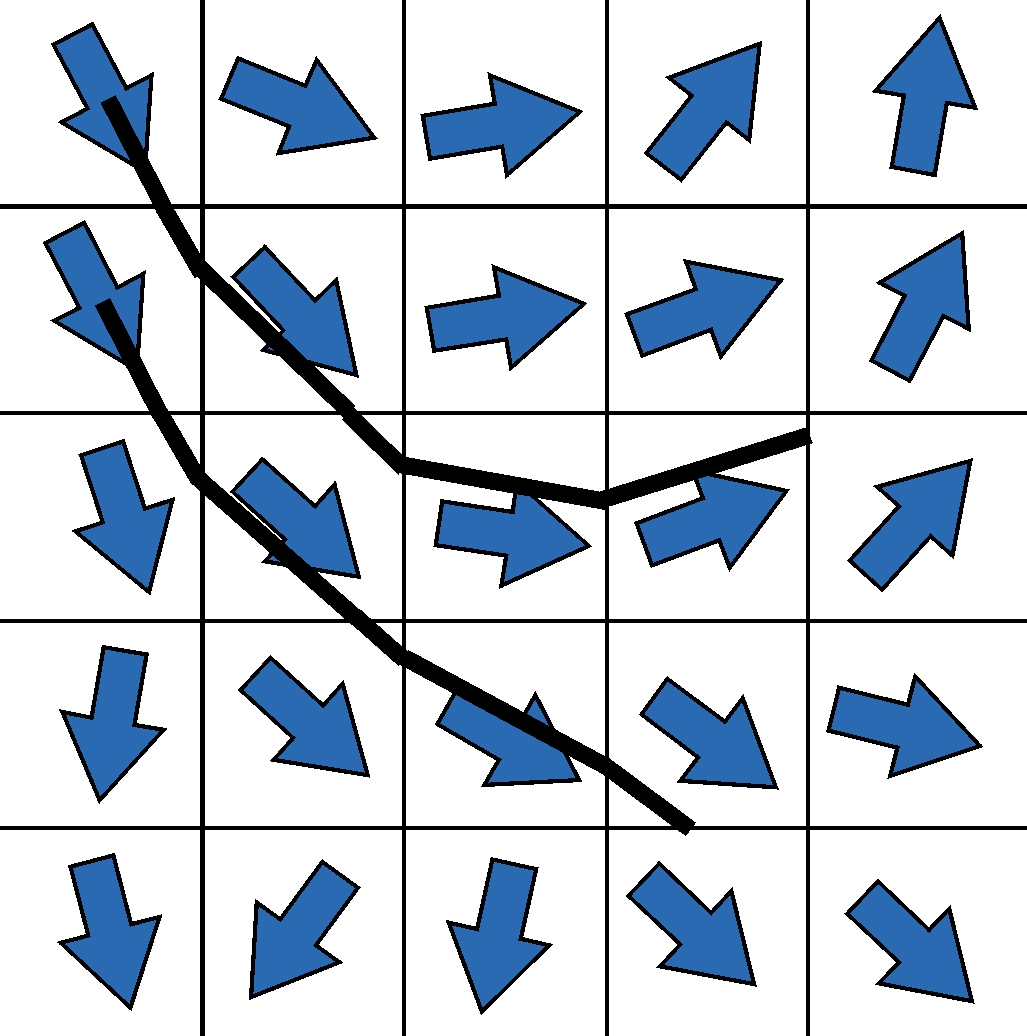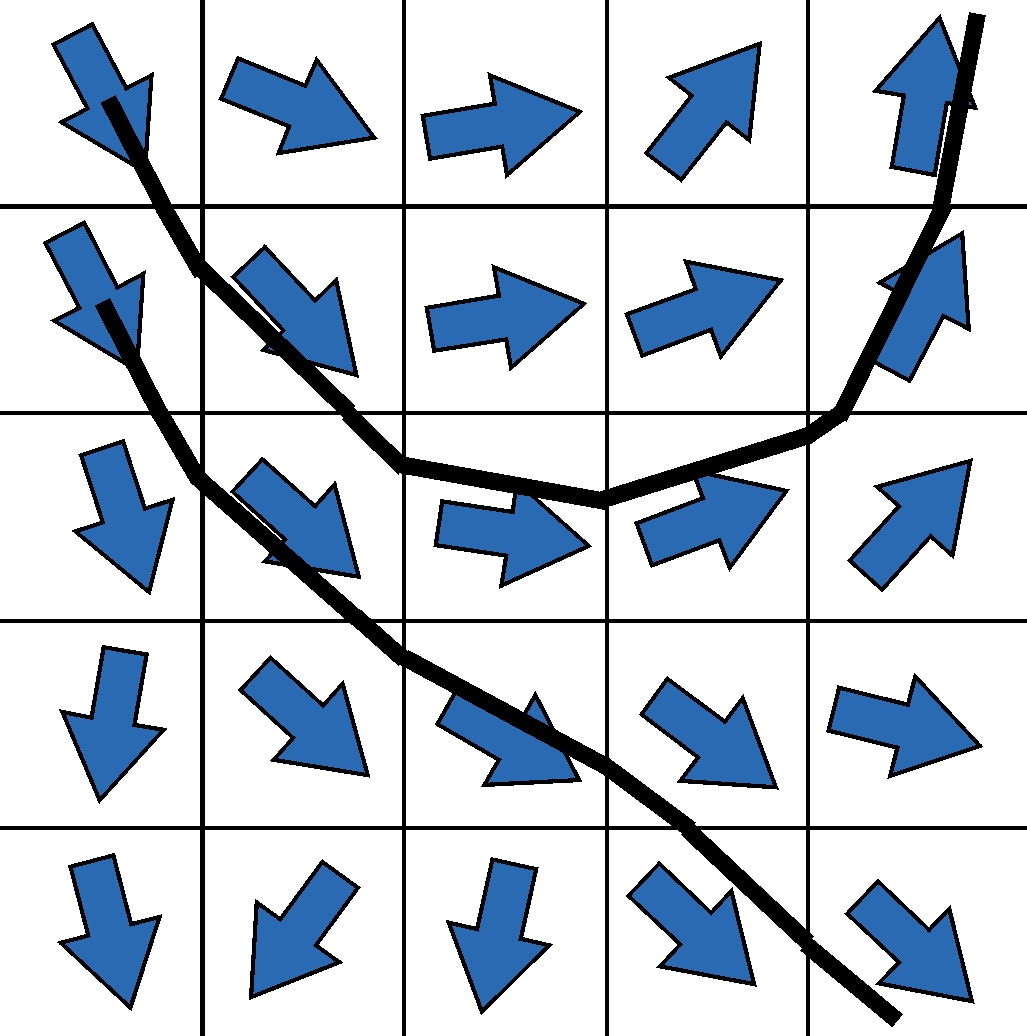 The calculation of the diffusion tensor as described above is the simplest possible form to determine directional information about the underlying fibers from the diffusion data for each image point. However, due to its intrinsical limitations, the tensor has many disadvantages in order to precisely resolve fiber connections, especially in complex geometric configurations such as crossing situations. In order to circumvent these limitations, a lot of research has been carried out in recent years to acquire more sophisticated data with more complex scanner hardware, more complex acquisition methods and more diffusion directions, which can then be reconstructed in a mathematically more complex fashion.
The calculation of the diffusion tensor as described above is the simplest possible form to determine directional information about the underlying fibers from the diffusion data for each image point. However, due to its intrinsical limitations, the tensor has many disadvantages in order to precisely resolve fiber connections, especially in complex geometric configurations such as crossing situations. In order to circumvent these limitations, a lot of research has been carried out in recent years to acquire more sophisticated data with more complex scanner hardware, more complex acquisition methods and more diffusion directions, which can then be reconstructed in a mathematically more complex fashion.
However, the idea of how to reconstruct fibers from tensor information is shown in two-dimensional form in the graphic on the left. The idea of the first tracking algorithms was to start in a voxel, reconstruct a piece of the nerve fiber in the direction of the main diffusion, and attach another piece to the next voxel edge, but now with the direction of the new main diffusion direction. This made it possible to reconstruct the first tractograms on which the main connections in the brain can be easily recognized. Corresponding developments in the last decade, as indicated above, lead to improved tractograms and reconstructions – and in the end also to our artistic BrainPics images.

